GLP-1 Drug Side Effect Risk Calculator
Calculate Your Side Effect Risk
Based on clinical data from 2025 trials, this tool assesses your risk for common GLP-1 side effects. Input your weight loss progress and medication details below.
When you hear about the latest wave of weight‑loss meds, the name next-generation GLP-1 agents is popping up everywhere. These drugs promise bigger drops in body weight, but they also bring new safety questions that clinicians and patients need to understand.
Why the New GLP-1 Wave Matters
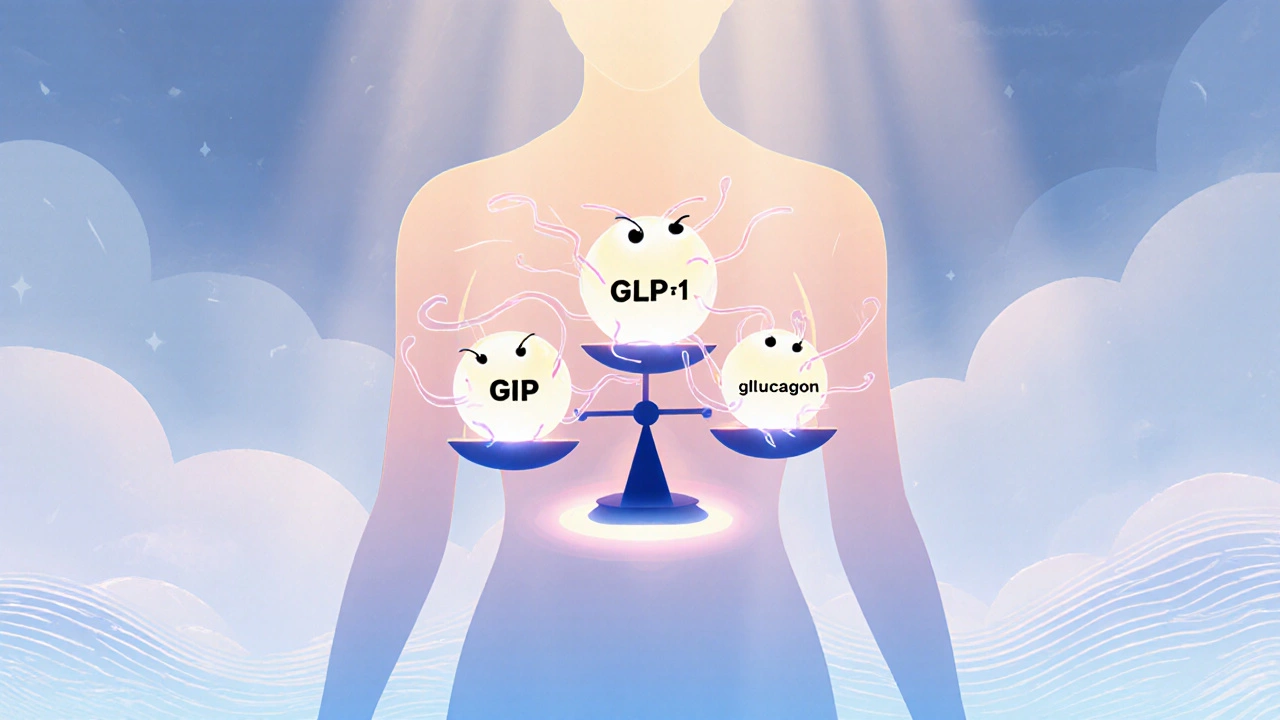
GLP-1 receptor agonists started as diabetes treatments, but the success of semaglutide for obesity opened the floodgates. The next generation builds on that foundation by adding extra hormone targets-GIP and glucagon-to squeeze out even more weight loss. The idea is simple: hit more pathways, get better results. The trade‑off? A more complex safety profile that isn’t fully mapped yet.
Key Players in the Pipeline
Here’s a quick run‑through of the agents that are furthest along in 2025:
- retatrutide - a triple agonist (GLP‑1, GIP, glucagon) from Eli Lilly. Phase III wraps up late 2025/2026.
- orforglipron - the first oral GLP‑1 agonist, being tested at 6‑36 mg doses.
- VK2735 - a GLP‑1/GIP dual agonist from Viking Therapeutics, with both injectable and oral forms in the pipeline.
- MET097 - an ultra‑long‑acting GLP‑1 RA that sticks around for weeks after a single shot.
- tirzepatide - already on the market as Mounjaro/Elsiglutide, serving as a benchmark for dual agonists.
How These Drugs Work Differently
All of them mimic the natural GLP‑1 hormone: they boost insulin, curb glucagon, slow stomach emptying, and tell the brain you’re full. The dual‑ and triple‑agonists go a step further by adding GIP or glucagon signals, which can improve energy expenditure and fat burning. That extra punch explains why retatrutide showed up to a 24 % weight loss in 48‑week trials, far beyond the 15‑20 % range of earlier drugs.

Safety Snapshot: What the Data Show
Gastrointestinal (GI) upset remains the most common complaint. In the 2025 ADA Standards, classic GLP‑1 agents cause nausea in 20‑35 % of patients, vomiting in 5‑15 %, and diarrhea in 10‑20 %. The newer multi‑agonists don’t seem to dodge these numbers. A PubMed study by Wen et al. (2025) found “no meaningful reduction in GI events” despite the added receptors.
Beyond the gut, several newer concerns surface:
- Musculoskeletal health: Rapid weight loss can shave off muscle. Dr. Daniel J. Drucker warns that >20 % loss may need strength‑preserving strategies.
- Pancreatitis risk: The AGA still flags it as a theoretical concern; long‑term data are thin.
- Cardiovascular & renal safety: Ongoing Phase III endpoints for retatrutide focus on heart and kidney outcomes.
Comparative Side‑Effect Table
| Agent | Nausea (%) | Vomiting (%) | Diarrhea (%) | Other notable AEs |
|---|---|---|---|---|
| semaglutide | 28 | 7 | 12 | Transient constipation (10 %) |
| tirzepatide | 30 | 10 | 15 | Increased heart rate (2 %) |
| retatrutide | 32 | 12 | 18 | Transient hypoglycemia (3 %) |
| orforglipron (oral) | 29 | 9 | 14 | Mild liver enzyme rise (1 %) |
| VK2735 | 27 | 8 | 13 | Injection site pain (5 %) |
Compounded GLP‑1 Products: A Safety Minefield
Not every GLP‑1 you see on a pharmacy shelf is FDA‑approved. The University of Illinois at Chicago’s Digital Pharmacy site warned in August 2025 that compounded versions can have “dosing inconsistencies, formulation variability, and serious adverse events.” Data show a 3‑5 × higher rate of severe reactions when non‑compliant compounding pharmacies are used. Bottom line: stick to FDA‑approved products whenever possible, and if you must use a compounded version, verify USP <795> compliance.
Practical Tips for Clinicians
- Start low, go slow. Titrate over 16‑20 weeks to the maintenance dose to blunt nausea and vomiting.
- Educate patients that GI symptoms usually improve after 4‑8 weeks of stable dosing.
- Monitor weight loss speed. If patients lose >1 % of body weight per week, add a protein‑rich diet and resistance‑training plan to protect muscle.
- Screen for pancreatitis signs (persistent abdominal pain, elevated lipase) at baseline and every 3‑6 months.
- For multi‑receptor agonists, order baseline cardiac echo and renal labs; repeat annually or sooner if symptoms appear.
- Document every dose change in the EMR and cross‑check with the pharmacy’s compounding record when applicable.
Future Outlook: Safety Will Drive the Next Wave
Industry analysts expect the GLP‑1 market to hit $120 billion by 2030. That money will fund more head‑to‑head safety trials. Eli Lilly’s retatrutide Phase III protocol explicitly measures bone density, muscle mass, and nutritional status over a five‑year period. Viking Therapeutics plans a head‑to‑head comparison of oral versus injectable VK2735 to see if the oral route truly eases GI intolerance.
Beyond obesity, researchers are testing GLP‑1 combos for heart failure, fatty liver disease, and even neurodegenerative disorders. Each new indication will bring its own safety checklist, reinforcing the need for robust post‑market surveillance.
Key Takeaways
- Next‑generation GLP‑1 agents deliver unprecedented weight loss but keep the same GI side‑effect profile as older drugs.
- Multi‑agonist therapy adds potential risks for muscle loss, bone health, and cardiovascular events-monitor them closely.
- Compounded products carry a higher danger signal; prefer FDA‑approved formulations.
- Slow titration, patient education, and regular labs are the simplest ways to stay safe.
What makes next‑generation GLP‑1 agents different from semaglutide?
They add extra hormone targets-usually GIP and sometimes glucagon-so they can boost energy expenditure and improve weight loss beyond what pure GLP‑1 agonists achieve.
Are the GI side effects worse with the newer drugs?
Data so far show similar rates of nausea, vomiting, and diarrhea. Titration speed is the biggest factor, not the number of receptors hit.
Should I prescribe a compounded GLP‑1 formulation?
Only if an FDA‑approved version is unavailable and the compounding pharmacy follows USP <795> standards. The risk of dosing errors is significantly higher with compounding.
How do I monitor muscle loss during rapid weight reduction?
Baseline DEXA or bioimpedance, combined with a structured resistance‑training program and adequate protein intake (1.2‑1.5 g/kg/day), helps preserve lean mass.
What long‑term safety data are missing?
We still need five‑plus‑year data on bone density, cardiovascular events, and renal outcomes for agents that cause >15‑20 % weight loss.

Sunita Basnet
October 26, 2025 AT 18:33Next‑gen GLP‑1 agonists are a paradigm shift in metabolic pharmacology
they harness GLP‑1, GIP and glucagon pathways to amplify anorectic signaling
the triple‑agonist mechanism raises basal metabolic rate and promotes adipose oxidation
clinical trials demonstrate up to 24% total body weight reduction over 48 weeks
the magnitude of loss surpasses legacy semaglutide outcomes by a wide margin
efficacy is driven by synergistic receptor activation and prolonged half‑life
pharmacokinetic profiling shows steady state after 6‑8 weeks of titration
the safety signal remains anchored to gastrointestinal tolerance
nausea, vomiting and diarrhea rates are comparable to earlier GLP‑1 agents
however, the broader receptor footprint introduces musculoskeletal considerations
rapid lean mass depletion can be mitigated with resistance training and protein supplementation
cardiovascular surveillance is essential given glucagon’s inotropic effects
renal function monitoring should be incorporated into standard protocols
patient education on gradual titration can blunt adverse events and improve adherence
overall the therapeutic benefit outweighs the manageable risk profile when used responsibly
Melody Barton
October 27, 2025 AT 22:20Listen up if you want real results – start low and push hard enough to hit the target dose fast but without causing a mess of nausea. The data are clear and the drugs work, so give them a solid try and don’t waste time hesitating.
Justin Scherer
October 29, 2025 AT 02:06Just a heads‑up – keep an eye on labs every few months, especially liver enzymes and kidney markers. It’s a good habit and it helps catch any weirdness early.
Pamela Clark
October 30, 2025 AT 05:53Oh great, another “breakthrough” that promises miracles while repeating the same old GI drama. As if we needed more nausea to go with our weight loss fantasies.
Diane Holding
October 31, 2025 AT 09:40Monitor muscle mass with DEXA; add resistance training; keep protein intake high.
Lionel du Plessis
November 1, 2025 AT 13:26From a pharmacology view the dual and triple agonists add GIP and glucagon signaling which can boost thermogenesis and improve energy expenditure while the GI side‑effects stay in the same ballpark as classic GLP‑1s
Leanne Henderson
November 2, 2025 AT 17:13Wow!!! This stuff really can change lives!!! Just remember to go slow on the dose, stay hydrated, and keep an open mind about the occasional stomach upset!!! It’s all part of the journey!!!
Megan Dicochea
November 3, 2025 AT 21:00These drugs are powerful but not a magic wand the key is consistent use and regular check‑ups the side effects are manageable with proper titration and lifestyle support
Suzanne Carawan
November 5, 2025 AT 00:46Sure, because we all love more nausea.